Explain the Difference Between Saturated and Unsaturated Hydrocarbons
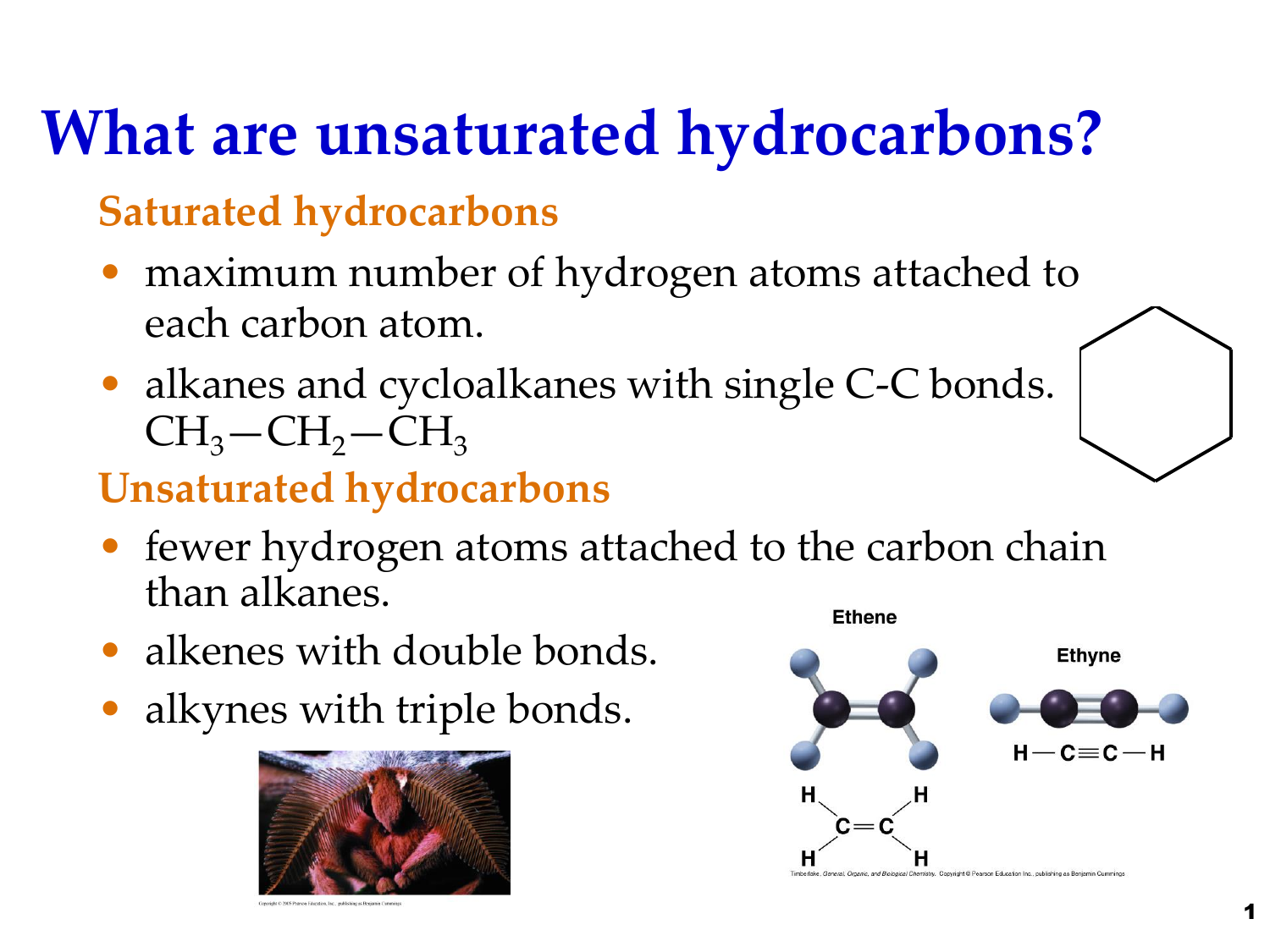
While saturated hydrocarbons only have a single bond between its hydrogen and carbon atoms unsaturated hydrocarbons have double and even triple bonds between the carbon atoms. In unsaturated hydrocarbons double bonds and triple bonds are also present.

Saturated Vs Unsaturated Hydrocarbons Labster Theory
A saturated hydrocarbon has all carbon-carbon single bonds while an unsaturated hydrocarbon has carbon-carbon multiple bonds.
. Unsaturated Hydrocarbons 2 Unsaturated Hydrocarbons Saturated Hydrocarbons contain only carbon-carbon single bonds. An unsaturated hydrocarbonhowever is the one in which some carbon-carbon bonds can be double or triple. Find step-by-step Chemistry solutions and your answer to the following textbook question.
Toluene is classified as an aromatic compound. Saturated hydrocarbons have a less amount of carbon atoms bonded to a high number of hydrogen. Eg alkanes like ethane propane.
A saturated hydrocarbon is the hydrocarbon in which all its carbon-carbon bonds are single. The saturated hydrocarbons are composed of single bonds whereas the unsaturated hydrocarbons are characterized by triple and double bonds. Explain the difference between saturated and unsaturated hydrocarbons.
Contain more hydrogen atoms than the corresponding unsaturated hydrocarbons. An unsaturated hydrocarbon is one wheresome of the carbon atoms arent an example being an alkene due. In saturated hydrocarbons all the bonds are single bonds.
Saturated and unsaturated hydrocarbons show different characteristics because of these. Unsaturated hydrocarbons are the straight chain compounds containing double or triple covalent bonds. Both unsaturated fat and saturated fat add calories to your meal and weight to your waistline if you consume too much.
It has the potential to react with bromine. They have Sp 3 hybridised carbon atoms and are called alkanes having a general formula C n H 2n2. It consists of a single bond between.
Examples include alkanes and cycloalkanes. This makes unsaturated hydrocarbons even more reactive than saturated hydrocarbons as well as have fewer hydrogen atoms bonded to the carbon atoms than. A saturated hydrocarbon is one where all the carbon atoms arebonded to four other atoms.
Saturated hydrocarbons have the highest number of hydrogen atoms carbon atoms can accommodate in contrast to. Practicing moderation is the best way to stay healthy. Explain the difference between saturated and unsaturated hydrocarbons.
This makes unsaturated hydrocarbons even more reactive than saturated hydrocarbons as well as have fewer hydrogen atoms bonded to the carbon atoms. Explain the difference between saturated hydrocarbons and unsaturated hydrocarbons. They contain sp 2 or sp hybridized carbons.
Thus unsaturated hydrocarbons are more reactive than saturated hydrocarbons and have fewer hydrogen atoms bonded to the carbon atoms than saturated hydrocarbons have. All carbon atoms are sp 3 hybridized in these compounds. Unlike saturated hydrocarbons in which all hydrogen atoms and carbon atoms are bonded together with single bonds unsaturated hydrocarbons have double or even triple bonds between the carbon atoms.
Draw the distinguishing structural feature for the following. Unsaturated Hydrocarbons contain carbon-carbon double or triple bonds more hydrogens can be added. C H H HC H H H Alkanes C H H C H H HCHC C C C C C C H H H H H H Alkenes Alkynes Aromatics.
These are the simplest hydrocarbons in which carbon-carbon atoms and carbon-hydrogen atoms are held together by single bonds. The physical properties are similar but chemical properties are different because of the variation of covalent bonds. Unlike saturated hydrocarbons in which all hydrogen atoms and carbon atoms are bonded together with single bonds unsaturated hydrocarbons have double or even triple bonds between the carbon atoms.
Saturated hydrocarbons after combustion give a clean flame while unsaturated hydrocarbons. Saturated hydrocarbons can undergo substitution reactions whereas the unsaturated hydrocarbons can undergo addition reactions. Additionally the type of fat-containing foods you consume can make a.
Show transcribed image text. Saturated hydrocarbons usually obtained from fossilized animals and plants whereas the unsaturated hydrocarbons usually obtained from plant materials. This problem has been solved.
Saturated hydrocarbons are the compounds containing only single covalent bonds. Previous question Next question. Ethane C 2 H 6 can also be written as CH 3 CH 3.
Contain fewer hydrogens than the corresponding saturated hydrocarbon. These are also known as alkanes. - saturated hydrocarbons have every available carbon bond beyond the single bonds between carbons filled with hydrogens - saturated hydrocarbons have only single bonds between carbons - unsaturated hydrocarbons have double andor triple bonds between carbons.
The main difference between saturated and unsaturated hydrocarbon is that saturated hydrocarbons contain only single covalent bonds between carbon atoms whereas unsaturated hydrocarbons contain at least one double or triple covalent bond in the main chain. Draw the structure of toluene CH. Explain the difference between saturated and unsaturated hydrocarbons.
Alkanes alkenes alkynes aromatics 3. Saturated hydrocarbons have a high amount of hydrogen whereas. See the answer See the answer See the answer done loading.
Explain the difference between saturated and unsaturated hydrocarbons. Toluene is the common name of methylbenzene. What is the difference between Saturated Hydrocarbons and Unsaturated Hydrocarbons.
5 rows Saturated hydrocarbon.

Give A Chemical Test To Distinguish Between Saturated And Unsaturated Hydrocarbon Edurev Class 10 Question

Difference Between Saturated And Unsaturated Hydrocarbon Javatpoint

Difference Between Saturated And Unsaturated Hydrocarbons Brainly In
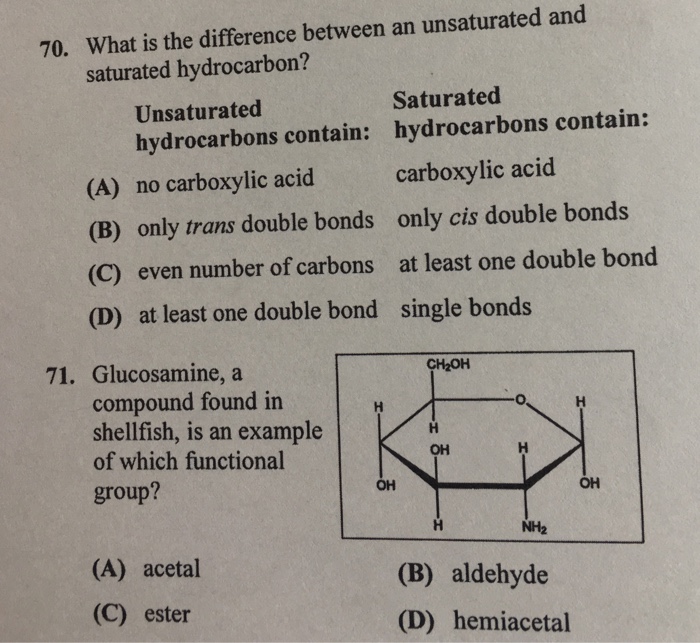
Solved What Is The Difference Between An Unsaturated And Chegg Com

Cnh2n 2 Is The General Formula Of A Homologous Series Of Hydrocarbons I Is This Series Saturated Or Unsaturated Ii Name The Series Described Above Give The Formula And Name Of The Member

What Is The Difference Between Saturated Hydrocarbons And Unsaturated Hydrocarbons Chemistry Youtube

Mention Four Differences Between Saturated And Unsaturated Hydrocarbons Snapsolve

Difference Between Saturated And Unsaturated Compounds

Saturated Hydrocarbon Unsaturated Hydrocarbon Definition Alkane Formula Properties Difference Cbse Ncert Class
Which Hydrocarbons Are Saturated Which Ones Are Unsaturated Quora
What Are Hydrocarbons Give Examples B Give The Structural Differences Between Saturated And Unsaturated Sarthaks Econnect Largest Online Education Community

List Two Differences Between Saturated And Unsaturated Hydrocarbons

स त प त ह इड र क र बन और अस त प त ह इड र क र बन

Give Difference Between Saturated And Unsaturated Hydrocarbons
Difference Between Saturated And Unsaturated Hydrocarbons Definition Structure Types Properties

Difference Between Saturated And Unsaturated Hydrocarbon Brainly In

Ncert Class X Science Class Chapter 4 Carbon And Its Compounds Part 12 Flexiprep
Difference Between Saturated And Unsaturated Hydrocarbons Definition Structure Types Properties
Comments
Post a Comment